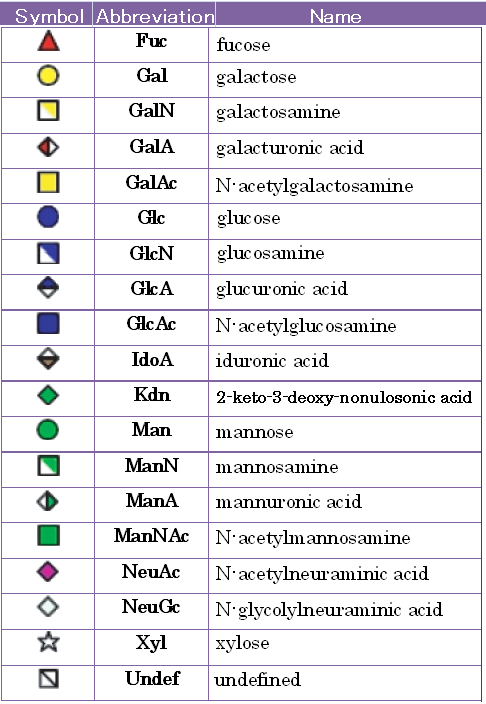
a) The standard representation of monosaccharides [1]
Since the basic building blocks of glycans are basically consistent for mammalian cells,
the Consortium for Functional Glycomics (CFG) has proposed a standard notation for glycans using symbols for commonly found monosaccharide residues.
This standard is provided in TABLE 2.1 and using throughout this tool and RINGS.
TABLE2.1 The standard representation of monosaccharides as proposed by the CFG. (cf. Glycome Informatics [1])
b) Classes of glycan core structure
[1]
Glycans are generally classified into classes based on the core structure of the glycan,
which is composed of the monosaccharides at the reducing end.
The major classes and their representative core structures are listed in TABLE 1.2.
N-glycans comprise the most commomly found glycans in mammalian systems and is made up of three major subclasses:
high-mannose, hybrid, and complex.
They are usually attached to core proteins via the R-group nitrogen (N) of asparagine residues, thus its name.
N-linked glycans are extremely important for proper folding of proteins in eukaryotic cells.
As described later, chaperone proteins participate in
N-glycan biosynthesis in order to ensure that proteins are properly folded.
Steric effects of
N-glycans also contribute to protein folding by blocking cysteine residues such that disulfide bonds are not formed, for example.
N-glycans also play various roles in cell-cell interactions and protein targetting,
but oftentimes it is not the core structure that is recognized, but the terminal structures, which may also be found on other types of glycans.
O-glycans are relatively smaller structures, usually attached to core proteins via serine or threonine residues.
Mucins are heavily
O-glycosylated glycoproteins,
containing VNTR (variable number of tandem repeat) regions that are rich in Ser / Thr acceptor sites of
O-glycans.
Other amino acids that may be
O-glycosylated include hydroxyproline found in plants, and hydroxylysine in collagens.
Glycosphingolipids are usually attached via ceramide residues.
In prokaryotes, a variety of glycoconjugates not found in mammalian systems exist,
including lipopolysaccharides (LPS), which consists of three parts:
a lipid A moiety embedded in the outer membrane,
a core oligosaccharide containing KDO and heptose which are monosaccharides that are not found in vertebrates,
and a polysaccharide side chain known as the
O-antigen.
Polysaccharides forming polymers may be considered another type of class whereby repeated structures (up to millions) of monosaccharide components form very large structures.
For example, cellulose fibers consisting of repeated β1-4Glc residues are coated and cross-linked with one another by glycans called hemicelluloses,
of which xyloglucan is the major representative found in the primary cell walls of most higher plants.
Chitin is another polymer consisting of GlcNAcβ1-4 residues and is considered the second most abundant biopolymer on Earth, next to cellulose.
It is a major component of the exoskelton of arthropods, and it is also found in the cell-wall of fungi as well as the cuticle of nematodes.
Peptidoglycans are another type of bacterial polymer constituting the major structural component of the periplasm.
it consists of MurNAcβ1-4GlcNAcβ1-4 repeat units, covalently cross-linked to short peptides.
TABLE2.2 (cf. Glycome Informatics [1])
![table2.1e.bmp(1026434 byte)]()
←2-1
2-3→



