a) Glycan structures [1]
Complex carbohydrates are composed of monosaccharides that are covalently linked by glycosidic bonds, either in the α or β forms.
Monosaccharides have the general empirical formula Cx(H20)n n={3, 4, …, 9},
usually forming a ring of carbon atoms numbered from C1, as in FIGURE 2.1.
The overall configuration (D or L) of each monosaccharide is determined by the absolute configuration of the stereogenic center furthest from the highest numbered asymmetric carbon atom,
which is C5 in hexoses and C4 in pentoses.
FIGURE 2.2 illustrates the D and L configurations of glucose.
Alternatively, the chiral center may be labeled as R or S, depending on the annotator.
Thus some databases will allow the definition of glycans using either the D / L or R / S systems.
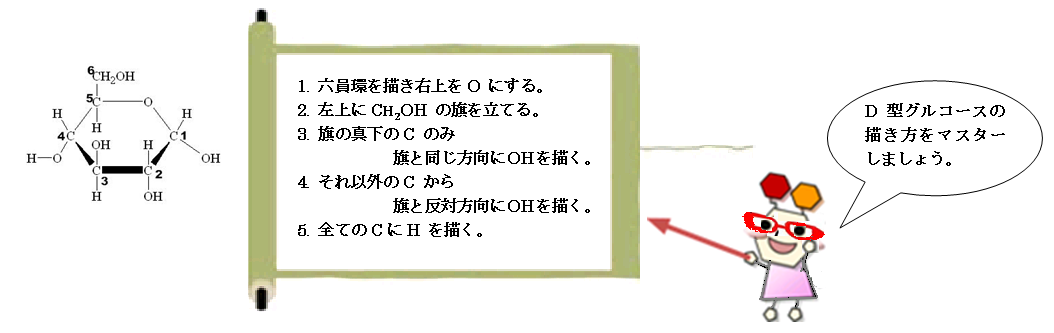
FIGURE2.1 Glucose monosaccharide with the carbon numbers numbered from C1 through C6 (cf. Glycome Informatics [1])
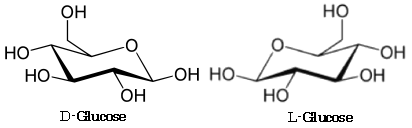
FIGURE2.2 D and L configurations of glucose (cf. Glycome Informatics [1])
Monosaccharides most commonly form five- or six-membered rings due to chemical stability.
A five-membered ring is called a pentose (or furanose), and a six-membered rind is called a hexose (or pyranose).
When cyclized into these rings, monosaccharides acquire an asymmetric center called the anomeric carbon
(the carbon atom having hemiacetal functionality, such as the C1 in the ring form of glucose as in FIGURE 2.1 or the C2 for sialic acids).
Two stereoisomers can be formed because the anomeric hydroxy group can assume two possible orientations.
When the anomeric carbon and the stereogenic center furthest away from it are the same, the monosaccharide is defined as the α anomer;
when they are different, it is defined as the β anomer.
Figure 2.3 illustrates how the anomeric configuration is determined.
These anomeric configurations may change when monosaccharides are joined to one another.
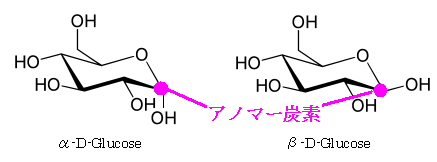
FIGURE2.3 The anomeric center is determined by the configuration of opposing carbon atoms. (cf. Glycome Informatics [1])
b) Disaccharides ・ polysaccharides
[1]
Two monosaccharide units are joined together by glycosidic bond,
which is formed between the anomeric carbon of one monosaccharide to a hydroxy group of another.
Unlike DNA and amino acid sequences, however, monosaccharides may be linked to more than one ather monosaccharide,
such that they form branched, tree structures.
For example, the chemical structure of the
N-linked glycan core structure is given in FIGURE 2.4.
This core structure infact consists of five building blocks of monosaccharides.
Thus glycans can be displayed with symbols representing each monosaccharide connected by glycosidic linkages as lines,
as in the corresponding FIGURE 2.5.
The dark shaded squares represent
N-acetylglucosamine (GlcNAc) residues and the shaded squares represent mannose residues.
The two GlcNAcs are linked in a β1-4 linkage, as well as the first βMan.
This Man is further linked to two other mannose residues by an α1-3 and an α1-6 linkage.
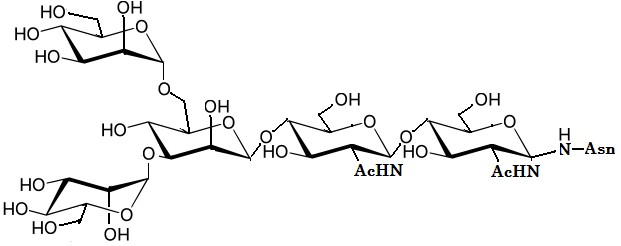
FIGURE2.4 The chemical structure of the N-glycan core structure. (cf. Glycome Informatics [1])
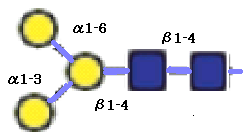
FIGURE2.5 The N-glycan core structure represented with monosaccharide symbols. (cf. Glycome Informatics [1])
2-2→







