※Coming soon!
a) Discovery of a great number of lectin
最初にレクチンが発見されてから今日まで、数々のレクチンが発見されてきました。
たくさんの情報を獲得することができるようになると、”分類”という方法を取るのが人間の特徴です。
また、分類といっても色々な方法があります。しかし、悪までも人間が決めるのですから、人間が研究するのに利用しやすいように分類するのが最善です。
まだ、発見されていないレクチンが多く存在しますが、多くのレクチンの一次構造が明らかにされているので、
どのレクチン分子が近縁であるかは客観的に判断できます。
また、ヒトをはじめとする多くの生物の全遺伝子構造が近い将来解読されようとしている時代においては、
レクチンもまずはタンパク質(遺伝子)familyとして整理していくのがベストでしょう。
実際には数々のレクチンの発見により、レクチンはそれらがもっとも強く結合する炭水化物配列により分類されています。
レクチンは、分子クローニングによって明らかになったアミノ酸配列の相似性と進化論的な近似性により、一貫したクラスが分類されるようになりました。
最初にこのようなクラス分類をしたのはDrickamerで、
彼は炭水化物結合ドメイン上の高度に保存されたアミノ酸配列モチーフに基づいてレクチンを2種類に大別しました。
一つのグループは、認識にカルシウムを必要とするので、C型レクチンと名付けました。
もう一つのグループは、遊離のチオール基を必要とするので、S型レクチンと名付けました。
一方、マンノース-6-リン酸を認識する二つのレクチンの配列が調べられ、両者の相同性が明らかになり、他のタイプのレクチンとは全く異なっていたため、
P型レクチンと名付けられました。
b) Lectin Family[1]
Lectins have been found in almost all classes and families of organisms.
Some mejor lectins are classified according to the glycan structures that they are known to recognize,
as listed in Table 3.1.
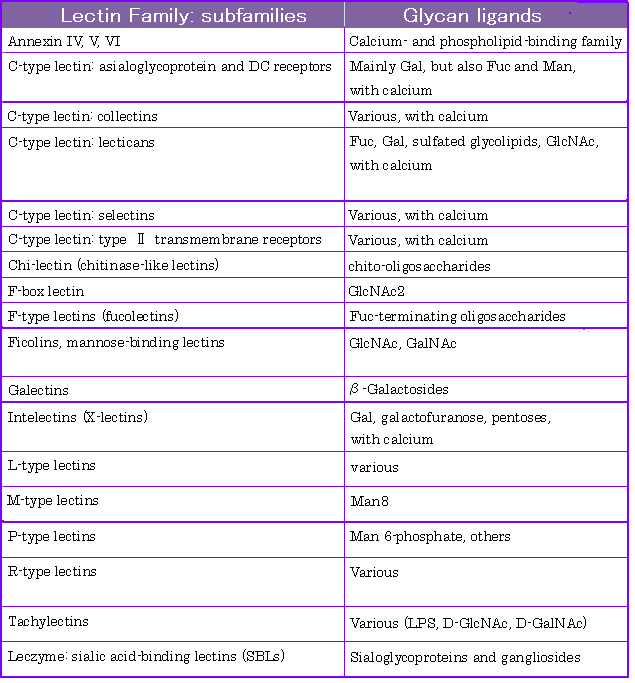
TABE3.1 レクチンファミリー (cf. Glycome Informatics[1])
※Annexin ・・・ multifunctional protein family binding to phospholipid or calcium.
Asialoglycoprotein ・・・ シアル酸を含む糖タンパク質からシアル酸を除去したもの
Collectins ・・・ host defense lectin
Selectins ・・・ レクチン様ドメインをもつ細胞接着タンパク質の総称
Ficolins ・・・ 重合体構造をもつレクチン
Galectins ・・・ β-ガラクトシド特異的に結合する動物レクチン
Tachylectins ・・・ カブトガニからとれるレクチン
c) Annexin
[4]

Annexins comprise a family of calcium-and phospholipid-binding proteins.
Over 20 members have been found in all eukaryotic kingdoms as well as plants and animals with the exception of fungi.
Annexins have molecular weights ranging between 30 and 40 kDa(only annexin VI is 66 kDa) and prossess striking structural features.
Annexins' aminoterminal domains are diverse in sequence and length(ranging from 11 to 196) on each annexin member.
In contrast the carboxyterminal regions consisting on 4(8 only for annexin VI) a-helical domains
composed of about 70 amino acid residues are well conserved among annexins.
The calcium- and phospholipid-binding sites are located in the carboxyterminal domains.
At least one of the members can be found expresses in nearly every eukaryotic cell types.
Almost all cells produce several kinds of annexins simultaniously and the expression levels are rather high.
Annexins bind to phosphatidylserine, phosphatidylethanolamine, and phosphatidylinositol,
which are known to be rich in the inner leaflet of plasma membrane and hardly appear on cell surface,
in contrast to phosphatidylcholin and sphingomyelin, which are major components of the outer leaflet of plasma membrane.
However, the latter they are not recognized by annexins.
It is reasonable to assume that the proposed functions of intracellular annexins
include regulation of phospholopase A2 activity due to sequesteration of substrate phospholopods,
and involvement in vesicular transport and observed on some intracellular annexins,
suggesting the active movement of annexins corresponding to dynamic lipid vesicle transport.
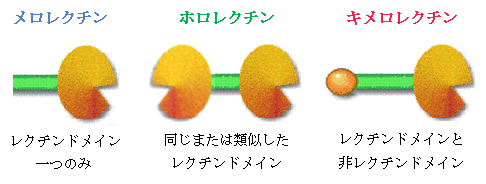
FIGURE3.1 Wide variety of function about annexin(GlycoWord[4]参照)
Schematic representation of proposed ligands for annexins in extracellular space.
Annexin Ⅱ binds to tissue plasminogen activator and tenascin.
Anchorin CⅡ isolated as a collagen-receptor is identical with annexin V.
Annexin IV,V,and VI exibit carbohydrate-binding activity,
however exact glycoconjugate ligands with phy siological relevance remaind to be elucidated.
d) C型レクチン
[4]
e) Galectin
[4]
Galectins are defined as lectins having both galactose-binding ability and amino-acid sequences which characterize galectins.
In general, galectins are soluble, and metal-independent in their activity.
They also hold many features of cytoplasmic proteins, ie., have no disulfide bridges, no sugar chains, no signal siquences,
and in most cases their N-terminal amino acids are acetylated.
However, their histological localization is diverse, not restricted in cytoplasm but also in nuclei,
on cell surfaces and in extracellular spaces, depending on the galectin species.
As regarde secretion, lottle is known, but a speculative model has been presented in which a classical signal sequence is not required.
Galectins show wide biological distribution not only in vertebrates but also in invertebrates, including nematodes, insects and sponges,
and more recently, in the fungus mushroom, the galectins of which have been proved to prossess galactose-binding activity.
A wide variety of biological phenomena have been shown to be related to galectins, e.g, development,
differentiation, morphogenesis, tumor metastasis, apotosis, RNA splicing, etc.
However, relatively little is known about the mechanism by which galectins exert these functions,
particularly in terms of carbohydrate recognition.
Galectins reported thus far can be classfied into three types on the basis of structural architecture,
i.e, proto, chimera and tandem-repeat types.
See below FIGURE3.1

FIGURE3.3 Three architectural types of galectin (GlycoWord[4]参照)
←3-1
3-3→






